Give The Difference Between Double Salt And Complex Salt To differentiate between double salt and complex salt Double salts are crystalline substances formed by the combination of two different salts through the process of crystallisation These
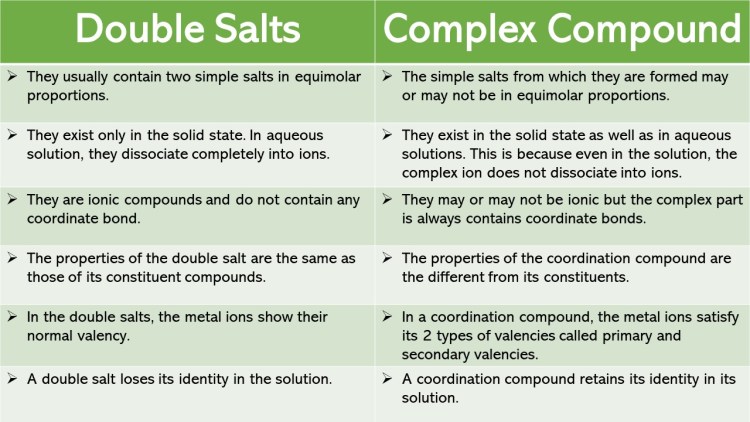
A double salt dissociates in water completely into simple ions whereas a coordination complex dissociates in water with at least one complex ion e g Mohr s salt FeSO 4 NH 4 2 SO 4 The aqueous solution of this salt gives the test of F e 2 N H 4 and S O 2 4 ions On the other hand complexes or coordination compounds retain their identity in aqueous solutions
Give The Difference Between Double Salt And Complex Salt

Give The Difference Between Double Salt And Complex Salt
https://i.ytimg.com/vi/TtUBvTxdYEY/maxresdefault.jpg

Difference Between Double Salt And Complex Salt Ifdiff
https://ifdiff.com/wp-content/uploads/2024/06/Difference-Between-Double-Salt-and-Complex-Salt.jpg

Difference Between Double Salt And Complex Salt YouTube
https://i.ytimg.com/vi/5_10xwmsq6M/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLAKMc9Uxtl4WMBBzQGtqXGvdlUJOg
Double salts are ionic compounds that are formed by the combination of two different salt compounds Complex salts are ionic compounds that contain a central metal atom which is Double salts contain two different cations or anions and dissociate completely in solution whereas complex salts contain a complex ion and do not dissociate entirely in solution
1 The nature of bonds in a double salt is conic or electrovalent It does not consist of covalent or co ordinate bonds 2 The physical and chemical properties or double salt are similar to their Complex salts are compounds where a central metal atom is surrounded by other molecules or ions forming a coordination complex In contrast double salts are combinations
More picture related to Give The Difference Between Double Salt And Complex Salt

Differences Between Double Salt And Complex Compound YouTube
https://i.ytimg.com/vi/Un4MUqR_W8w/maxres2.jpg?sqp=-oaymwEoCIAKENAF8quKqQMcGADwAQH4Ac4FgAKACooCDAgAEAEYSCBeKGUwDw==&rs=AOn4CLCN-k-cNLOKmDOQpoQ_PeBr-2UyJw

Difference Between Double Salt And Complex Salt 1 1 Point
https://i.ytimg.com/vi/ibI3ruQbY3M/maxresdefault.jpg

Difference Between Double Salt And Complex Compound Hindi Class 12
https://i.ytimg.com/vi/XuzQixeEbDM/maxresdefault.jpg
The primary difference between double salts and complex salts is the type of chemical bonding that holds the salts together Double salts are held together by ionic bonds between the individual ions whereas complex salts are held When two salts are crystallised together in stoichiometric ratio from their saturated solution to form double salts In complex salts it is made up of a central metal atom or ion which used to be
Complex salts are coordination compounds where a metal atom is surrounded by ligands coordinated through coordinate covalent bonds Double salts are combinations of two Double salts are formed from two different salts and dissociate completely in solution complex salts form coordination compounds and don t fully dissociate Double salts

Double Salt And Complex Salt Coordination Compounds Chemistry
https://i.ytimg.com/vi/nekbp8lZIjM/maxresdefault.jpg

Double Salt And Complex Compound Coordination Chemistry 01
https://i.ytimg.com/vi/kng2RfLpyl0/maxresdefault.jpg

https://www.vedantu.com › jee-main › chemistry...
To differentiate between double salt and complex salt Double salts are crystalline substances formed by the combination of two different salts through the process of crystallisation These

https://www.shaalaa.com › question-bank-solutions › ...
A double salt dissociates in water completely into simple ions whereas a coordination complex dissociates in water with at least one complex ion e g Mohr s salt FeSO 4 NH 4 2 SO 4

Difference Between Double Salt And Complex Salt Brainly in

Double Salt And Complex Salt Coordination Compounds Chemistry

Difference Between Double Salt And Coordination Salt YouTube

Difference Between Double Salts And Complex Salts L 2 Learning

Difference Between Double Salt And Complex Salt Definition
Coordination Compounds And Double Salts NCERT MCQ

Coordination Compounds And Double Salts NCERT MCQ
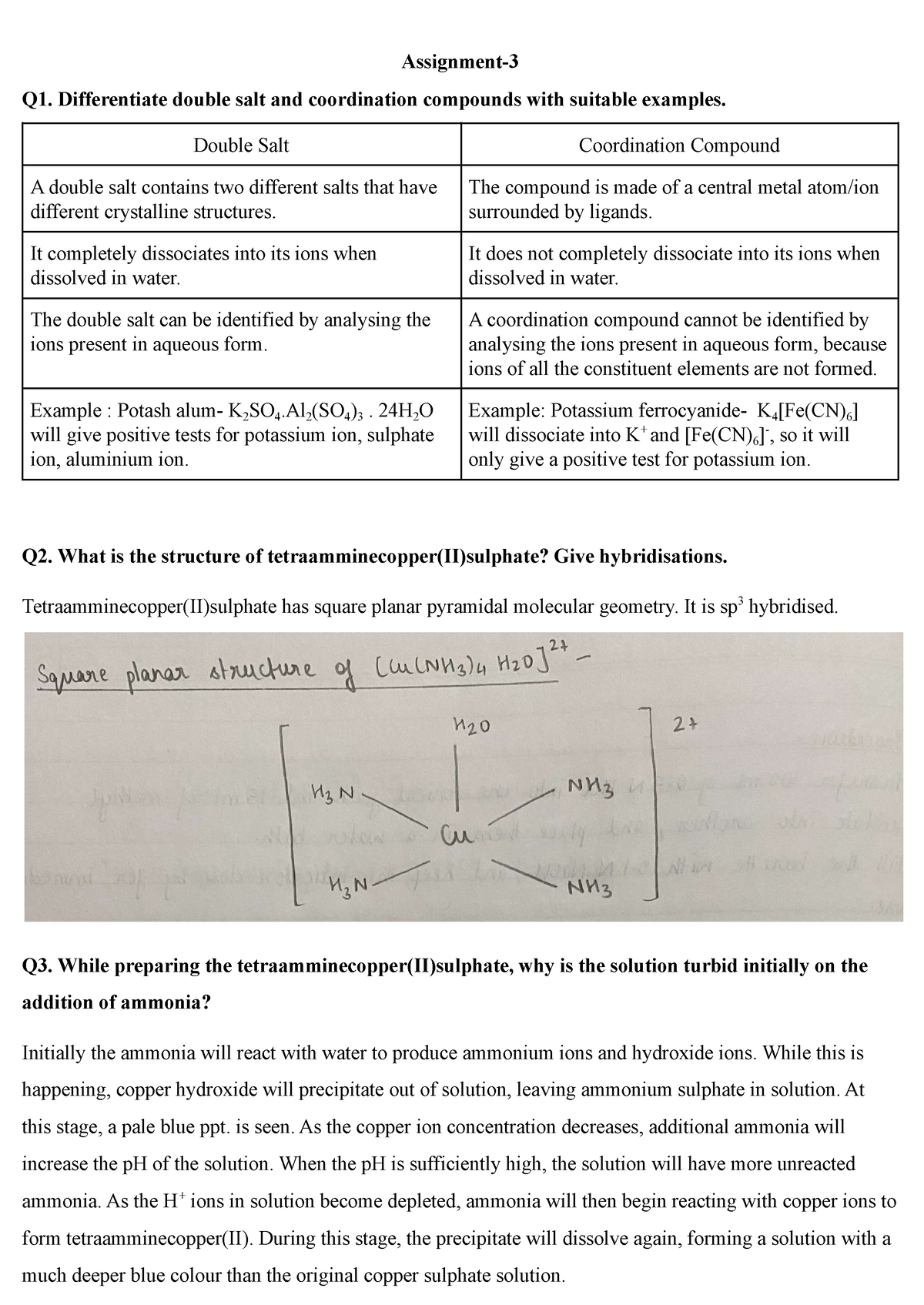
Nitika 115 Assignment 3 Assignment Q1 Differentiate Double Salt And

Difference Between Double Salt And Complex Salt Chemistry Class 12

Difference Between Double Salts Mixed Salt And Complex Salts Of Class
Give The Difference Between Double Salt And Complex Salt - 1 Double salts exist only in the solid state and dissociate into their constituent ions in the aqueous solutions 1 Coordination compounds exist in the solid state as well as in the