Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026 If a multi dose has been opened or accessed e g needle punctured the vial should be dated and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial
If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be used expiration date and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial The Joint Commission requires organizations to relabel multi dose vials with a revised expiration date of 28 days once the vial is opened or punctured Print a Free 28 Day Expiration Calculator Labels are White with Red text Material Vinyl Quantity 5 per package Dimensions 14 W x 2 H
Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026

Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026
https://cdn.menmd.com/wp-content/uploads/2019/09/09145643/SyringeandVile-01-1536x539.jpg

Multi Dose Vial Label 1 Dia United Ad Label
https://www.unitedadlabel.com/media/catalog/product/cache/57580a20c0160842cc537cb359690ccc/U/L/ULPH229.jpg
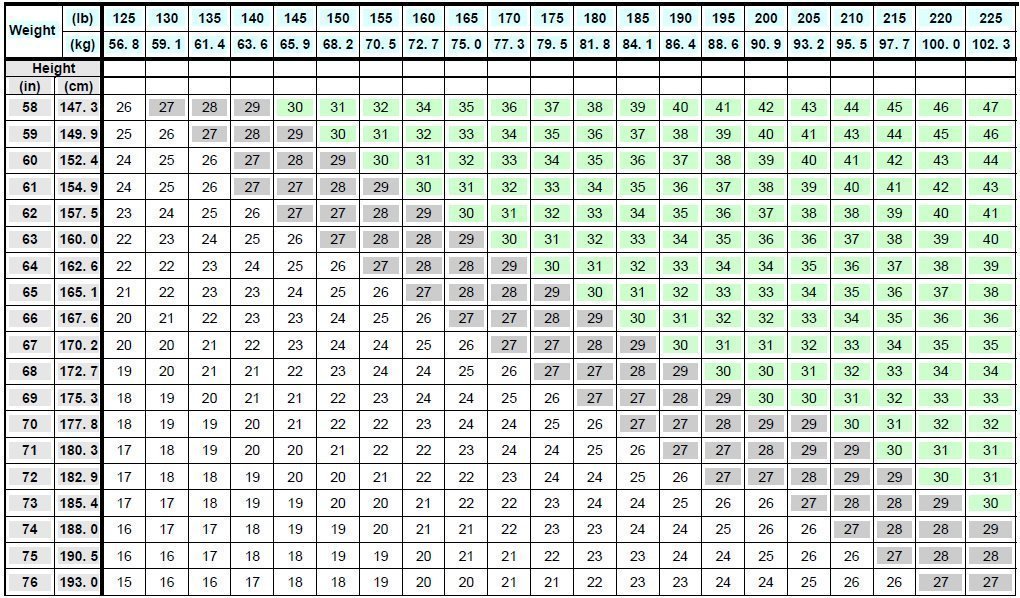
Saxenda FDA Prescribing Information Side Effects And Uses
http://www.drugs.com/pro/images/3946d389-0926-4f77-a708-0acb8153b143/image-07.jpg
Therefore The Joint Commission requires a 28 day expiration date for multi dose vials from the date of opening or puncture unless otherwise specified by the manufacturer The 28 day time frame is based on the fact that manufacturers are required by law to test the effectiveness of the bacteriostatic agent used in the Multi dose vials are dated by healthcare when they are first opened and discarded within 28 days unless the manufacturer specifies a diferent shorter or longer date for that opened vial Note This is diferent from
28 days after vial puncture or expiration date whichever is earlier Jun 2023 updated each flu season Flucelvax quadrivalent cell cultured inactivated influenza vaccine ccIIV4 Seqirus No BUD use until stamped expiration date unless visibly contaminated Jun 2023 updated each flu season Fluzone With reuse of single dose vials and misuse of multiple dose vials As a result of these incidents patients have su ered significant harms including death CDC and the One Only Campaign urge healthcare providers to recognize the di erences between single dose and multiple dose vials and to understand appropriate use
More picture related to Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026
Nationwide Shortage Of Tuberculin Skin Test Antigens CDC
https://www.cdc.gov/mmwr/volumes/68/wr/social-media/mm6824a4_TBTestChange_21June19_Image_1200x675.jpg

2025 2026 Proposed
https://www.weber.edu/wsuimages/registrar/pictures/2025-2026 Academic Calendar.png

Federal Register Medicare And Medicaid Programs CY 2015 Home Health
https://images.federalregister.gov/ER06NO14.009/original.png?1415261295
If the manufacturer s expiration date of recognized multi dose vials pens is prior to 28 days then the of Multi Dose Vials Pens of Injectable Medications and Vaccines in Acute Care and Ambulatory Care Environments Retrieved 01 29 2020 Official copy at http unchealthcare uncmc policystat policy 7112015 797 requires that the vial be dated to re ect the date opened and or beyond use date On the other hand the Centers for Disease Control and Prevention CDC indicates that multi dose vials can be used until the manufacturer s expiration date unless there are any concerns regarding the sterility of the product 15
SDV and Multi Dose Vials MDV July 2013 purposes It is not to be considered as medical legal or other professional contains general information only and may not reflect the most current the information contained herein and Contact David Chen Director Pharmacy Practice Sections and Section of Pharmacy Practice Multi Dose Vial 28 Day Expiration Calculator ePAPER READ DOWNLOAD ePAPER TAGS december february january october april november august september expiration vial calculator gohcl Create successful ePaper yourself Turn your PDF publications into a flip book with our
Pfizer COVID Vaccine Vials May Hold Extra Doses Adding To US Supply
https://www.gannett-cdn.com/presto/2020/12/17/USAT/30f1e0d4-244c-4490-b380-8e14bc2ba249-AP20351760766153.jpg?crop=1999,1125,x0,y0&width=1999&height=1125&format=pjpg&auto=webp

Health Care Logistics Multi Dose Vial Labels W Expiration Date Right
https://rightwaymed.com/wp-content/uploads/2021/01/HCL18369-min.png

https://www.cdc.gov/.../provider_faqs_multivials.html
If a multi dose has been opened or accessed e g needle punctured the vial should be dated and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial

https://www.jointcommission.org/standards/standard...
If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be used expiration date and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial

Project Firstline Resources For Multi Dose Vial Safety ANA

Pfizer COVID Vaccine Vials May Hold Extra Doses Adding To US Supply

White DATE VIAL OPENED Expiration Labels United Ad Label

Thiamine Hydrochloride Injection USP

Vial Of Life Form PDF Fill Out And Sign Printable PDF Template SignNow

Tips For Pharmacists Wegovy semaglutide Injection 2 4 Mg

Tips For Pharmacists Wegovy semaglutide Injection 2 4 Mg

Fluzone Flu Vaccine 5 ML Multi Dose Vial 10 Doses Per Vial Merit

Multi Dose Vial 28 Day Expiration Graphics Printable Calendar

Multi Dose Vial 28 Day Expiration Calculator Image Calendar
Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026 - With reuse of single dose vials and misuse of multiple dose vials As a result of these incidents patients have su ered significant harms including death CDC and the One Only Campaign urge healthcare providers to recognize the di erences between single dose and multiple dose vials and to understand appropriate use