Printable 28 Day Multi Dose Vial Expiration Calendar 2025 If a multi dose has been opened or accessed e g needle punctured the vial should be dated and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial
If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be used expiration date and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial 18511 The Joint Commission requires organizations to relabel multi dose vials with a revised expiration date of 28 days once the vial is opened or punctured Print a Free 28 Day Expiration Calculator Labels are White with Red text Material Vinyl Quantity 5 per package Dimensions 14 W x 2 H
Printable 28 Day Multi Dose Vial Expiration Calendar 2025

Printable 28 Day Multi Dose Vial Expiration Calendar 2025
https://rightwaymed.com/wp-content/uploads/2021/01/HCL18369-min.png

Multi Dose Vial Label 1 Dia United Ad Label
https://www.unitedadlabel.com/media/catalog/product/cache/57580a20c0160842cc537cb359690ccc/U/L/ULPH229.jpg

Project Firstline Resources For Multi Dose Vial Safety ANA
https://www.nursingworld.org/~49c632/globalassets/project-firstline/on-the-go-resources/youtube-screenshots/episode_8b.png
The Joint Commission requires organizations to re label multi dose vials with a revised expiration date beyond use date once a multi dose vial is opened or punctured according to the article USP and APIC now recommend that opened or punctured multi dose vials be used for no more than 28 days unless Multi dose vials are dated by healthcare when they are first opened and discarded within 28 days unless the manufacturer specifies a diferent shorter or longer date for that opened vial Note This is diferent from
With reuse of single dose vials and misuse of multiple dose vials As a result of these incidents patients have su ered significant harms including death CDC and the One Only Campaign urge healthcare providers to recognize the di erences between single dose and multiple dose vials and to understand appropriate use Normally 28 days unless manufacture label specifies otherwise or evidence of visible contamination Date and discard within 28 days unless manufacture label specifies CDC safe injection practices state MDVs should be dedicated to a single patient as much as possible If MDVs are used for more than one patient they must not
More picture related to Printable 28 Day Multi Dose Vial Expiration Calendar 2025
Pharmacists Deciding What To Do With Extra Vaccine Doses Left In Multi
https://media.tegna-media.com/assets/WPMT/images/e10c1e33-4d73-4f36-bf2c-d087ad50df7e/e10c1e33-4d73-4f36-bf2c-d087ad50df7e_1920x1080.jpg
Project Firstline Resources For Multi Dose Vial Safety ANA
https://www.nursingworld.org/~497e21/globalassets/project-firstline/alt2.png
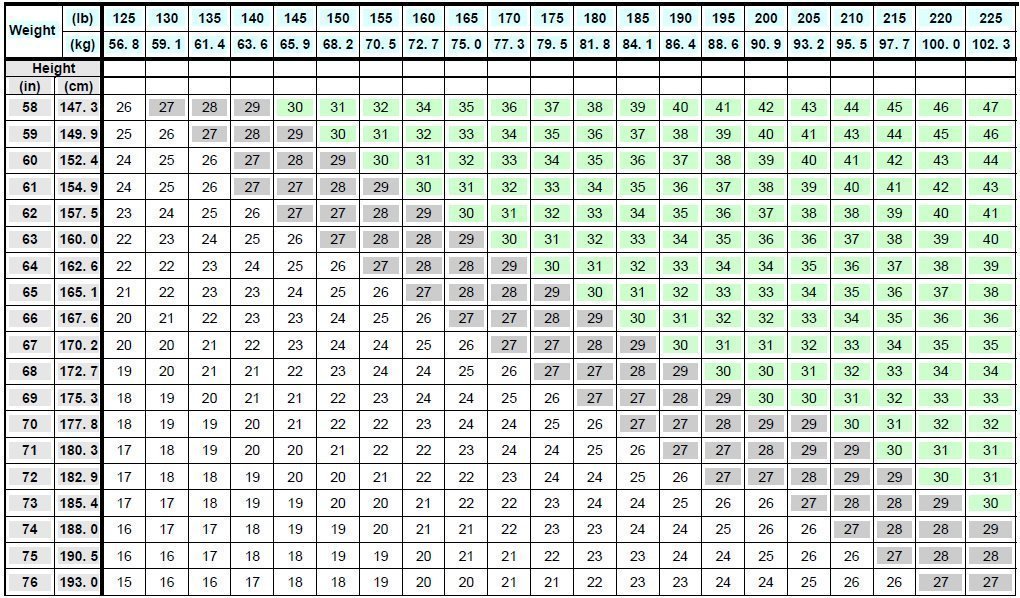
Saxenda Dosage Guide Drugs
https://www.drugs.com/pro/images/3946d389-0926-4f77-a708-0acb8153b143/image-07.jpg
797 requires that the vial be dated to re ect the date opened and or beyond use date On the other hand the Centers for Disease Control and Prevention CDC indicates that multi dose vials can be used until the manufacturer s expiration date unless there are any concerns regarding the sterility of the product 15 Multi Dose Vial 28 Day Expiration Calculator DATE OPENED 2013 February 1 March 1 February 2 March 2
Procedure Practitioners should discard multi dose vials when Suspected contamination has occurred Contents are discolored or have otherwise altered in appearance 28 days after opening has passed unless otherwise specified by the manufacturer The manufacturer s expiration date on the vial or pen has passed What should I do with expired vaccines Storage Handling Vaccine Viability and Expiration Is it acceptable to write the expiration date the Beyond Use Date of an opened vaccine multidose vial on the box rather than the vial or must it be written on the vial Storage Handling Vaccine Viability and Expiration
Nationwide Shortage Of Tuberculin Skin Test Antigens CDC
https://www.cdc.gov/mmwr/volumes/68/wr/social-media/mm6824a4_TBTestChange_21June19_Image_1200x675.jpg

Custom Accepted Pp Sustanon250mg ml Injection Usp 10ml Sterile Multiple
https://img.alicdn.com/imgextra/i1/6000000001531/O1CN019PQpru1NBEtfd3bvl_!!6000000001531-0-tbvideo.jpg

https://www.cdc.gov/.../provider_faqs_multivials.html
If a multi dose has been opened or accessed e g needle punctured the vial should be dated and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial

https://www.jointcommission.org/standards/standard...
If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be used expiration date and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial
SUCCINYLCHOLINE CHLORIDE INJECTION USP 200mg 10mL 20mg mL 10mL VIAL

Nationwide Shortage Of Tuberculin Skin Test Antigens CDC

Thiamine Hydrochloride Injection USP
Multi Dose Vial Safety Reminders For National Immunization Awareness

Federal Register Medicare And Medicaid Programs CY 2015 Home Health
Afluria Quadrivalent Flu Vaccine 2022 2023 Multi Dose Vial 1 X 5 ML

Afluria Quadrivalent Flu Vaccine 2022 2023 Multi Dose Vial 1 X 5 ML

28 Days Forecast 28daysavail With GENREPPAR FMX

White DATE VIAL OPENED Expiration Labels United Ad Label

Tips For Pharmacists Wegovy semaglutide Injection 2 4 Mg
Printable 28 Day Multi Dose Vial Expiration Calendar 2025 - Date the multi dose vial when first opened discard within 28 days unless the manufacturer recommends a shorter expiration period Wear a facemask when placing a catheter or injecting material into the epidural or subdural space such as during myelogram epidural or spinal anesthesia