Multi Dose Vial 28 Day Expiration Calendar 2025 Printable If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be
28 days after vial puncture or expiration date whichever is earlier Jun 2023 updated each flu season Flucelvax quadrivalent cell cultured Date the multi dose vial when first opened discard within 28 days unless the manufacturer recommends a shorter expiration period Wear a facemask when
Multi Dose Vial 28 Day Expiration Calendar 2025 Printable

Multi Dose Vial 28 Day Expiration Calendar 2025 Printable
https://rightwaymed.com/wp-content/uploads/2021/01/HCL18369-min.png

Project Firstline Resources For Multi Dose Vial Safety ANA
https://www.nursingworld.org/~49c632/globalassets/project-firstline/on-the-go-resources/youtube-screenshots/episode_8b.png

Multi Dose Vial Label 1 Dia United Ad Label
https://www.unitedadlabel.com/media/catalog/product/cache/57580a20c0160842cc537cb359690ccc/U/L/ULPH229.jpg
Therefore The Joint Commission requires a 28 day expiration date for multi dose vials from the date of opening or puncture unless otherwise Multi dose vials are dated by healthcare when they are first opened and discarded within 28 days unless the manufacturer specifies a diferent shorter or longer
Multi Dose Vial 28 Day Expiration Calculator ePAPER READ DOWNLOAD ePAPER TAGS december february january october april Normally 28 days unless manufacture label specifies otherwise or evidence of visible contamination Date and discard within 28 days unless manufacture label
More picture related to Multi Dose Vial 28 Day Expiration Calendar 2025 Printable
Project Firstline Resources For Multi Dose Vial Safety ANA
https://www.nursingworld.org/~497e21/globalassets/project-firstline/alt2.png
Saxenda Dosage Guide Drugs
https://www.drugs.com/pro/images/3946d389-0926-4f77-a708-0acb8153b143/image-07.jpg
Pharmacists Deciding What To Do With Extra Vaccine Doses Left In Multi
https://media.tegna-media.com/assets/WPMT/images/e10c1e33-4d73-4f36-bf2c-d087ad50df7e/e10c1e33-4d73-4f36-bf2c-d087ad50df7e_1920x1080.jpg
Convenient 28 Day Multi Dose Vial Expiration Date Assigner Labels allow your pharmacy team to know that date at quick glance Once identified add With the exception of COVID 19 vaccines vaccines in multidose vials MDVs that do not require reconstitution contain preservatives and can be
A multi dose vial is a vial of liquid medication intended for injection or infusion that contains more than one dose of medication Currently authorized Always prepare multi dose vial injections away from patient care spaces in a clean designated area Clean your hands before touching the vial Check the label to
Multi Dose Vial Safety Reminders For National Immunization Awareness
https://blogs.cdc.gov/safehealthcare/wp-content/uploads/sites/11/2023/08/vial800.jpg
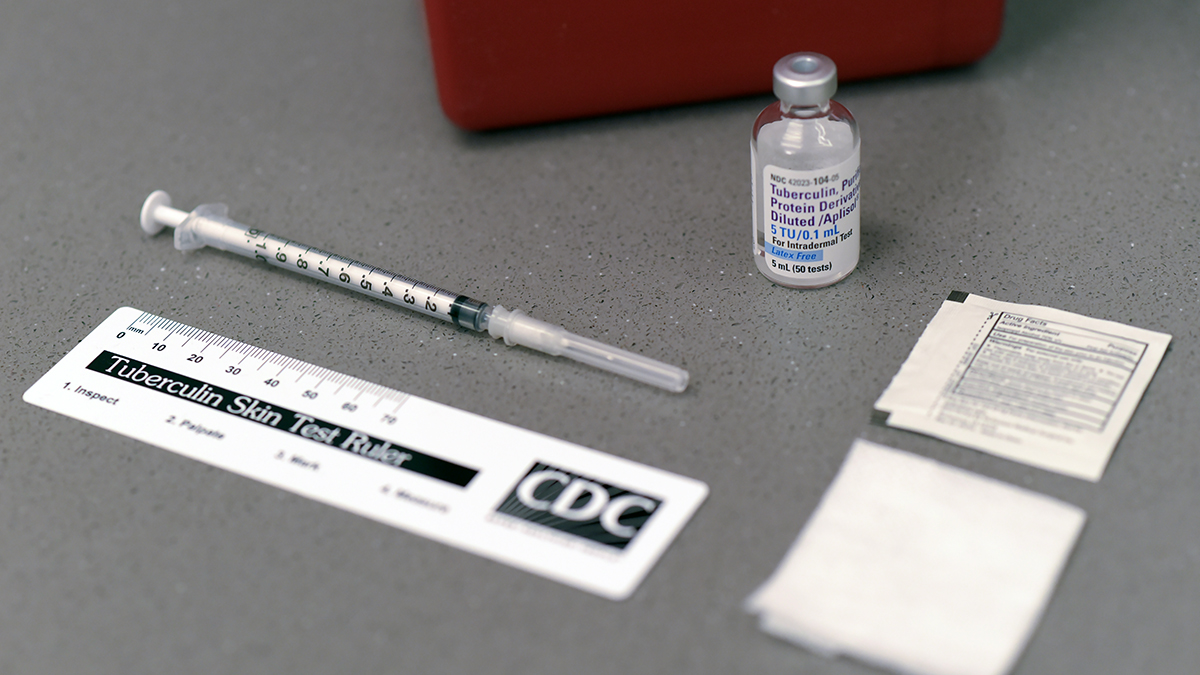
Nationwide Shortage Of Tuberculin Skin Test Antigens CDC
https://www.cdc.gov/mmwr/volumes/68/wr/social-media/mm6824a4_TBTestChange_21June19_Image_1200x675.jpg

https://www.jointcommission.org/standards/standard...
If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be

https://vce.health.mil/MHSHome/Reference-Center/...
28 days after vial puncture or expiration date whichever is earlier Jun 2023 updated each flu season Flucelvax quadrivalent cell cultured
Demo Dose Mini Vial 2 Ml Nasco Healthcare

Multi Dose Vial Safety Reminders For National Immunization Awareness

Custom Accepted Pp Sustanon250mg ml Injection Usp 10ml Sterile Multiple

Thiamine Hydrochloride Injection USP

Federal Register Medicare And Medicaid Programs CY 2015 Home Health

Demo Dose Empty Vials 10 Ml Nasco Healthcare

Demo Dose Empty Vials 10 Ml Nasco Healthcare
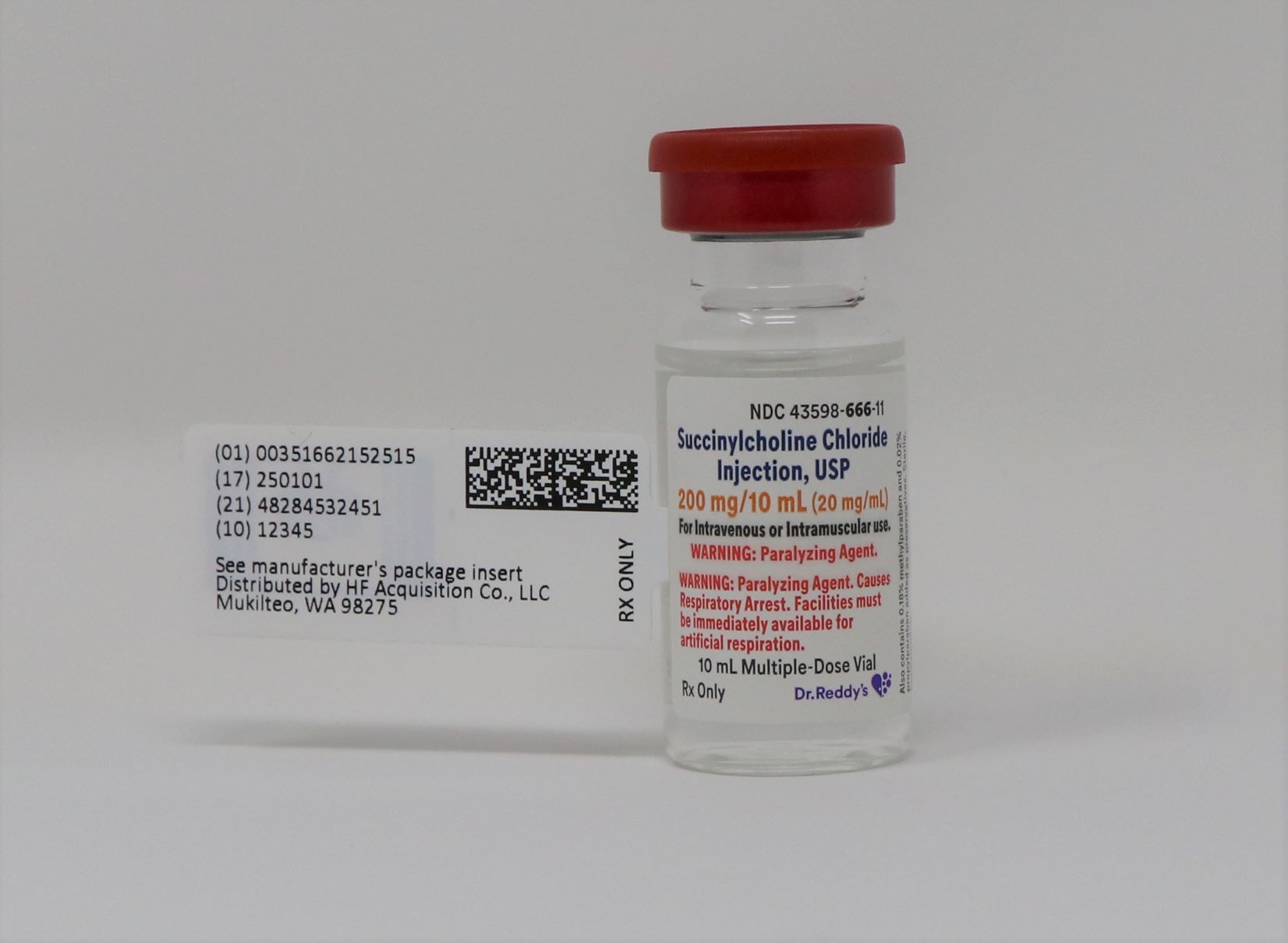
SUCCINYLCHOLINE CHLORIDE INJECTION USP 200mg 10mL 20mg mL 10mL VIAL

Multi Dose Vial Safety Reminders For National Immunization Awareness

White DATE VIAL OPENED Expiration Labels United Ad Label
Multi Dose Vial 28 Day Expiration Calendar 2025 Printable - Therefore The Joint Commission requires a 28 day expiration date for multi dose vials from the date of opening or puncture unless otherwise